 Mini Review
Mini Review
Scientific Studies on Momordica Charantia L.
Mohammad Kamil*
Director General, Lotus Holistic Health Institute, Abu Dhabi, UAE
Mohammad Kamil, Director General, Lotus Holistic Health Institute, Abu Dhabi, UAE.
Received Date:April 13, 2023; Published Date:April 19, 2023
Introduction
Momordica charantia L., commonly known as Bitter Melon or Bitter Gourd (Carilla fruit) belongs to the family Cucurbitaceae. A monoecious plant cultivated thought India up to an altitude of 1,500 m Figure [1]. The roots are bitter, acrid, astringent and ophthalmic, and are useful in colpoptosis and ophthalmopathy. The leaves are bitter, anthelmintic, antipyretic, emetic, and purgative. Helminthiasisul in vitiating conditions of pitta, helminthiasis, constipation, intermittent fever, burning sensation of the sole and nyctalopia. The fruits are bitter, acrid, thermogenic, depurative, vulnerary, stimulant, purgative, appetizing, antidiabetic, carminative, digestive, stomachic, anthekmintic, anti-inflammatory, emmenagogue, febrifuge and tonic. They are useful in vitiated conditions of kapha and pitta, skin diseases, leprosy, ulcer, wounds, burning sensation, constipation, anorexia, flatulence, colic, helminthiasis, eheumatalgia, gout, diabetes, hepatomegaly, splenomegaly, haemorrhoids, inflammatin, asthma, cough, dysmenorrhoea, impurity of breast milk, fever and debility. Seeds are useful in the treatment of ulcers, pharyngodynia and obstructions of the liver and spleen. The leaves and fruits are used for external application in lumbago, ulceration and bone fractures and internally in leprosy, haemorrhoids and jaundice. A lot of studies have already been carried out with special reference to antidiabetic activity. The present paper deals with pharmacognostic, phytochemical, pharmacological and toxicological studies on the root, leaves and fruits of Momordia charantia.

Pharmacognosy & Phytochemistry:
Plant Material of Interest : Root, leaves and fruits (whole
plant)
General Appearance : Leaf blade, 5+12 cm long and
broad, renifarm or suborbicular, fruits muricate, tuberculate,
oblong.
Organoleptic Properties : Free flowing powder is green in
colour having a characteristic odour and bitter taste.
Microscopic characteristics : NA
Powdered plant material : NA
General identity test : Characteristic chromatographic
finger printing.
Chemical:
Foreign organic matter : NA
Total ash : NMT 5%
Acid insoluble ash : NA
Water soluble extractive : NA
Alcohol soluble extractive : NA
Loss on drying : NA
Swelling index : NA
Pesticide residues : DNA
Heavy metals : Arsenic NMT 1 ppm, Lead
NMT 5ppm
Radioactive residues: : NA
Other purity tests: : NA
Chemical assay : Assay of active principle by
HPTLC/ HPLC, NLT 30% w/w
Major Chemical Constituents: 5-stigmasta-7, 25-dein-3--ol. Charautin, Momordicin, Momordicoside-I, cryptoxanthin, Elasterol, Flavochromone, Pectin (1) Table [1,2,3].
Table 1:Effect of Momordica charantia (Aqueous extract) on BP and HR of normotensive anaesthetized Wistar Rats.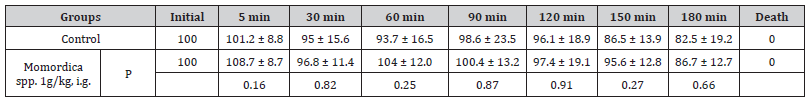
% Change of Systolic blood pressure (mean ± SD n=6)
Table 2:% Change of Diastolic blood pressure (mean ± SD, n=6).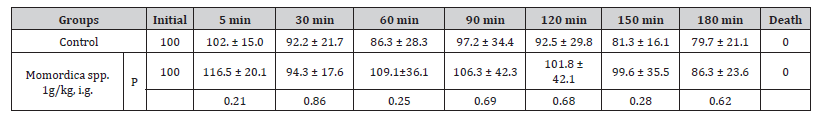
Table 3:% Change of Heart rate (mean ± SD, n=6)
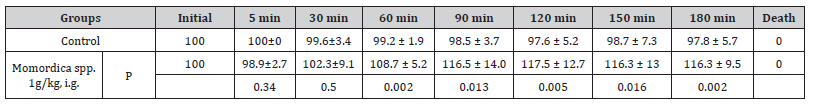
Extract : Momordica water extrac
Animal : Wistar rats, both males and females
Protocol : the rats were anesthetized with Urethane at 1.2g/
kg, ip; carotid canulationn Table [4].

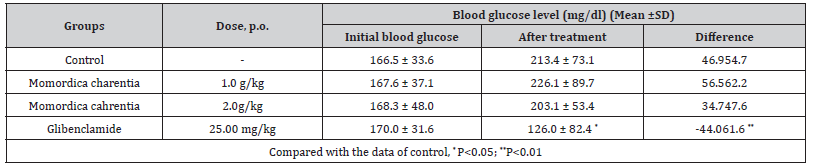
Difference : between the ‘initial blood glucose’ and the ‘blood
glucose after treatment’
Extract : water extract of Momordica charantia,
Animals : male T/O mice, body weight 18-25g.
Protocol : mice were divided into four groups and fasted for
12 hours. Momordica spp. (1g/kg and 2g/kg) and glibenclamide
(25mg/kg) were administered to treated groups and water to
control group. Ninety minutes later, glucose solution was given
orally at 2g/kg. Blood was collected from retro-orbital plexus
before the drug administration and 30 minutes after the glucose
loading Table [5].
Difference: between the ‘Initial Blood Glucose’ and the ‘Blood
Glucose After Treatment’
Extract: water extract of Momordica charantia,
Animals: T/O mice, both males and females. STZ was injected
(100mg/kg, ip) 12 days ago to cause diabetes.
Protocol: the diabetic mice were divided Fasting time was 4
hours. Fasting blood glucose was tested 3 hours after the drugs’
administration.
There were no significant toxicological symptoms at the dose tested. The data suggest that the hypoglycemic properties of bitter melon (Momordica charantia, Linn. Family, Cucurbitaceae), are at least partially due to their sulfonylurea-like activity.
The Acute Toxicity of Momordica charantia on Mice
Table 6:The effects of Momordica charantia (10g/kg, p.o) on hematology and body weight in mice (X±SE, n=10)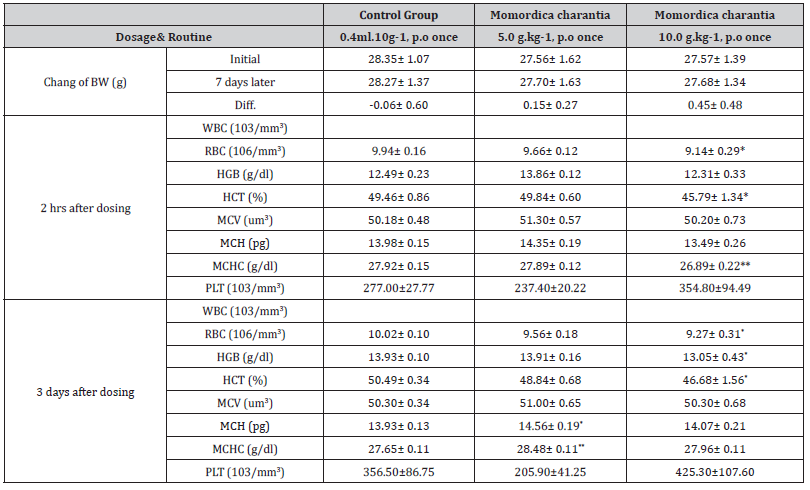
The Acute Toxicity of Momordica charantia on Mice
Table 7:The effects of Momordica charantia extract (10g/kg, p.o) on hematology and body weight in mice (X±SE, n=10)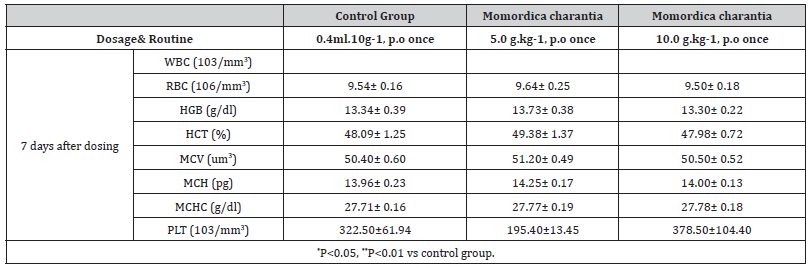
Animals: MF mice, both of male and female were used.
Extracts: Momordica charantia (water-extract was dissolved in
dilution water before they were used.
Dosage and Routine: Momordica charantia) 5.0-10 g.kg-1was
given orally, control group was treated with equal volume dilution
water (0.4 ml.10g-1).
Signs and symptoms of observation: 30 minutes after extracts
was given orally, the signs and symptoms of animal behavior was
checked, such as loco motor activity, aggressive behavior, diarrhea,
ataxia, string, platform and pole test, lasted to 4 hrs and then 7
days. The changes of body weight in each group were checked and
compared. Hematology was checked 2 hrs, 3 days and 7 days after
extract was given with Animal Blood Counter.
Results: The change of body weight and result of hematology
is as follows (Table):
Conclusion: 20% of mice showed slight weakness in platform
and pole test 1 hr after 10 g.kg-1 of MT combination was given.
But it recovered to normal 4 hrs after dosing. RBC and Hgb were
also lower in this group 2 hrs after dosing and lasted 3 days. There
are not any side effects when 5.0 g.kg-1 water-extracts of MT
combination was given orally; the LD50 of Momordica charantia is
more than 10 g.kg-1 (p.o).
The Effects of Momordia charantia on Body Weight in Mice
Table 8:The effects of Momordica charantia extract (10g/kg, p.o) on hematology and body weight in mice (X±SE, n=10)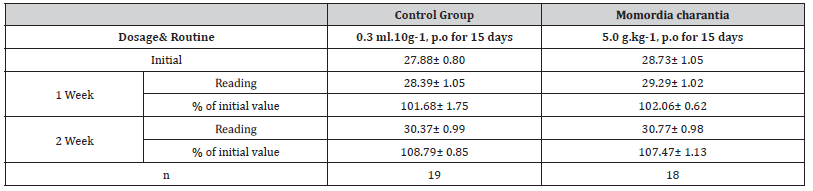
Animals: T/O mice, both of male and female were used.
Extract: Momordica (water-extract, was dissolved in dilution
water before they were used.
Dosage and Routine: Momordia charantia 5.0g.kg-1was given
orally, control group was treated with equal volume dilution water
(0.3 ml.10g-1), total is 15 days.
Signs and symptoms of observation: the signs and symptoms
of animal behavior were checked every day, such as loco motor
activity, aggressive behavior, diarrhea after extracts were given
orally, for 10 days. The changes of body weight in each group were
checked and compared in 1 and 2 weeks.
Results The change of body weight is as follows (Table):
Conclusion: There are not any effects to the behavioral activity, no effects to the change of body weight when 5.0 g.kg-1 waterextracts of Momordia charantia was given orally for 15 days Table [9].
Table 9:The effects of Momordica charantia on prothrombin time in mice in mice (Mean ± SE).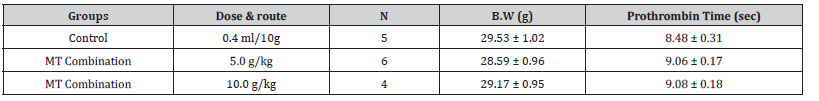
Animals: T/O mice, both of male and female were used.
Extracts: Momordica (Aqueous extract),
Dose regime: 5.0 –10.0 g/kg of Momordica charantia
Protocol: 2 hrs after extracts was given orally, 9 vol. blood
was collected in 1 vol. 3.2% trisodium citrate, blood sample was
centrifuged for 10 min at 2,500 g. plasmas were collected for
prothrombin time test with diagnostic stago instrument
Results: Momordica charantia (Aqueous extract) at the
dose 5-10 g/kg administered orally, once, showed no effect to
prothmobin time in mice.
The Effect of Momordica extract on locomotor activity in Mice
Table 10:The effects of Momordica extract (5.0g/kg, p.o) on locomotor activity in mice (TESTING TIME: 30 minutes; X±SE).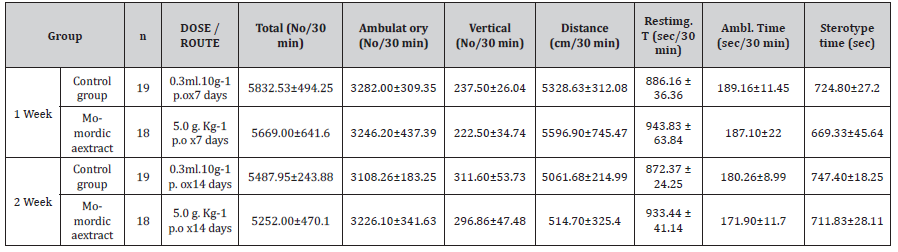
Animals: T/O mice, both of male and female were used.
Extracts: Momordica water-extract, was dissolved in dilution
water before use.
Dosage and Routine: 5.0 g.kg-1 was given orally for 15 days.
Animal model: Locomotor activity (software with computer
was supplied by Cloblous Co. USA)
Procedure: the locomotor activity (including total numbers,
ambulatory numbers, vertical numbers, distance, resting time,
ambulatory time, sterotype time) of mice in box was recorded in 30
minutes in 1 and 2 weeks.
Conclusion: there were no effects to locomotor activity in mice
when 5.0 g.kg-1 of MT water extracts was given orally for 15 days.
Acknowledgement
Thanks are due to M T Abdallah for technical support.
Conflict of Interest
None.
References
- (1989) Medicinal plants in China, WHO Regional Publications, WHO, Manila.
- Vasistha SK, Antony TC (1960-61) Chem. Exam. of Mom. Chara. Part I, J. Sci. Res. B.H. U. 12 (2): 228.
- Chen Q, Chan LL, Li ET (2003) Bitter melon (Momordica charantia) reduces adiposity, lowers serum insulin and normalizes glucose tolerance in rats fed a high fat diet. J Nutr 133(4): 1088-1093.
- Kar A, Choudhary BK, Bandyopadhyay NG (2003) Comparative evaluation of hypoglycaemic activity of some Indian medicinal plants in alloxan diabetic rats. J Ethnopharmacol 84(1): 105-108.
- Nagasawa H, Watanabe K, Inatomi H (2002) Effects of bitter melon (Momordica charantia l.) or ginger rhizome (Zingiber offifinale rosc) on spontaneous mammary tumorigenesis in SHN mice. Am J Chin Med 30(2-3): 195-205.
- Kohler I, Jenett-Siems K, Siems K, Hernandez MA, Ibarra RA, et al. (2002) In vitro antiplasmodial investigation of medicinal plants from El Salvador. Z Naturforsch [C] 57(3-4): 277-81.
-
Mohammad Kamil*. Scientific Studies on Momordica Charantia L.. Arch Phar & Pharmacol Res. 3(4): 2023. APPR.MS.ID.000566.
-
Momordica charantia, Ophthalmopathy, Purgative, Leprosy, Haemorrhoids, Jaundice, Diabetes, Asthma, Cough
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






